So the question that arises on the occasion of the Sound Science Working Paper is:
Can it be that the Covid tests were actually used to diagnose “Mendelian Diseases”?
What is “Mendelian Diseases” supposed to be? My last information about this kind of “diagnosis” of “diseases” goes back to Hitler, the Nazis and social Darwinism.
The Eugenics Record Office at Cold Spring Harbor, 1910-1940: an Essay in Institutional History
Garland E. Allen, Washington University in St Louis, GAllen@WUSTL.EDU
Follow this and additional works at: https://openscholarship.wustl.edu/bio_facpubs
https://openscholarship.wustl.edu/cgi/viewcontent.cgi?article=1140&context=bio_facpubs
Biology Faculty Publications & Presentations | Biology | Washington University in St. Louis
Part of the Biology Commons, Genetics Commons, History of Science, Technology, and
Medicine Commons, and the United States History Commons Recommended Citation
Allen, Garland E., „The Eugenics Record Office at Cold Spring Harbor, 1910-1940: an Essay in Institutional History“ (1986). Biology Faculty Publications & Presentations. 141.
https://openscholarship.wustl.edu/bio_facpubs/141
The Definition of Gene Therapy Has Changed
A single-gene disorder (or monogenic disorder) is the result of a single mutated gene. Single-gene disorders can be passed on to subsequent generations in several ways. Genomic imprinting and uniparental disomy, however, may affect inheritance patterns. The divisions between recessive and dominant types are not „hard and fast“, although the divisions between autosomaland X-linked types are (since the latter types are distinguished purely based on the chromosomal location of the gene). For example, the common form of dwarfism, achondroplasia, is typically considered a dominant disorder, but children with two genes for achondroplasia have a severe and usually lethal skeletal disorder, one that achondroplasics could be considered carriers for. Sickle cell anemia is also considered a recessive condition, but heterozygous carriers have increased resistance to malaria in early childhood, which could be described as a related dominant condition.[18] When a couple where one partner or both are affected or carriers of a single-gene disorder wish to have a child, they can do so through in vitro fertilization, which enables preimplantation genetic diagnosis to occur to check whether the embryo has the genetic disorder.[19]
Most congenital metabolic disorders known as inborn errors of metabolism result from single-gene defects. Many such single-gene defects can decrease the fitness of affected people and are therefore present in the population in lower frequencies compared to what would be expected based on simple probabilistic calculations.[20]
Autosomal dominant
Main article: Autosomal dominant § Autosomal dominant gene
Only one mutated copy of the gene will be necessary for a person to be affected by an autosomal dominant disorder. Each affected person usually has one affected parent.[21]: 57 The chance a child will inherit the mutated gene is 50%. Autosomal dominant conditions sometimes have reduced penetrance, which means although only one mutated copy is needed, not all individuals who inherit that mutation go on to develop the disease. Examples of this type of disorder are Huntington’s disease,[21]: 58 neurofibromatosis type 1, neurofibromatosis type 2, Marfan syndrome, hereditary nonpolyposis colorectal cancer, hereditary multiple exostoses(a highly penetrant autosomal dominant disorder), tuberous sclerosis, Von Willebrand disease, and acute intermittent porphyria. Birth defects are also called congenital anomalies.[22]
Autosomal recessive
Main article: Autosomal dominant § Autosomal recessive allele
Two copies of the gene must be mutated for a person to be affected by an autosomal recessive disorder. An affected person usually has unaffected parents who each carry a single copy of the mutated gene and are referred to as genetic carriers. Each parent with a defective gene normally do not have symptoms.[23] Two unaffected people who each carry one copy of the mutated gene have a 25% risk with each pregnancy of having a child affected by the disorder. Examples of this type of disorder are albinism, medium-chain acyl-CoA dehydrogenase deficiency, cystic fibrosis, sickle cell disease, Tay–Sachs disease, Niemann–Pick disease, spinal muscular atrophy, and Roberts syndrome. Certain other phenotypes, such as wet versus dry earwax, are also determined in an autosomal recessive fashion.[24][25] Some autosomal recessive disorders are common because, in the past, carrying one of the faulty genes led to a slight protection against an infectious disease or toxinsuch as tuberculosis or malaria.[26] Such disorders include cystic fibrosis,[27] sickle cell disease,[28] phenylketonuria[29] and thalassaemia.[30]

There are well over 6,000 known genetic disorders,[4] and new genetic disorders are constantly being described in medical literature.[5]More than 600 genetic disorders are treatable.[6]Around 1 in 50 people are affected by a known single-gene disorder, while around 1 in 263 are affected by a chromosomal disorder.[7] Around 65% of people have some kind of health problem as a result of congenital genetic mutations.[7] Due to the significantly large number of genetic disorders, approximately 1 in 21 people are affected by a genetic disorder classified as „rare“ (usually defined as affecting less than 1 in 2,000 people). Most genetic disorders are rare in themselves.[5][8]
Genetic disorders are present before birth, and some genetic disorders produce birth defects, but birth defects can also be developmental rather than hereditary. The opposite of a hereditary disease is an acquired disease. Most cancers, although they involve genetic mutations to a small proportion of cells in the body, are acquired diseases. Some cancer syndromes, however, such as BRCA mutations, are hereditary genetic disorders.[9]
https://en.m.wikipedia.org/w/index.php?title=Genetic_disorder&diffonly=true#Single_gene_dosierter
- Hereditary defects in enzymes are generally inherited in an autosomal fashion because there are more non-X chromosomes than X-chromosomes, and a recessive fashion because the enzymes from the unaffected genes are generally sufficient to prevent symptoms in carriers.

- On the other hand, hereditary defects in structural proteins (such as osteogenesis imperfecta, Marfan’s syndrome and many Ehlers–Danlos syndromes) are generally autosomal dominant, because it is enough that some components are defective to make the whole structure dysfunctional. This is a dominant-negative process, wherein a mutated gene product adversely affects the non-mutated gene product within the same cell.
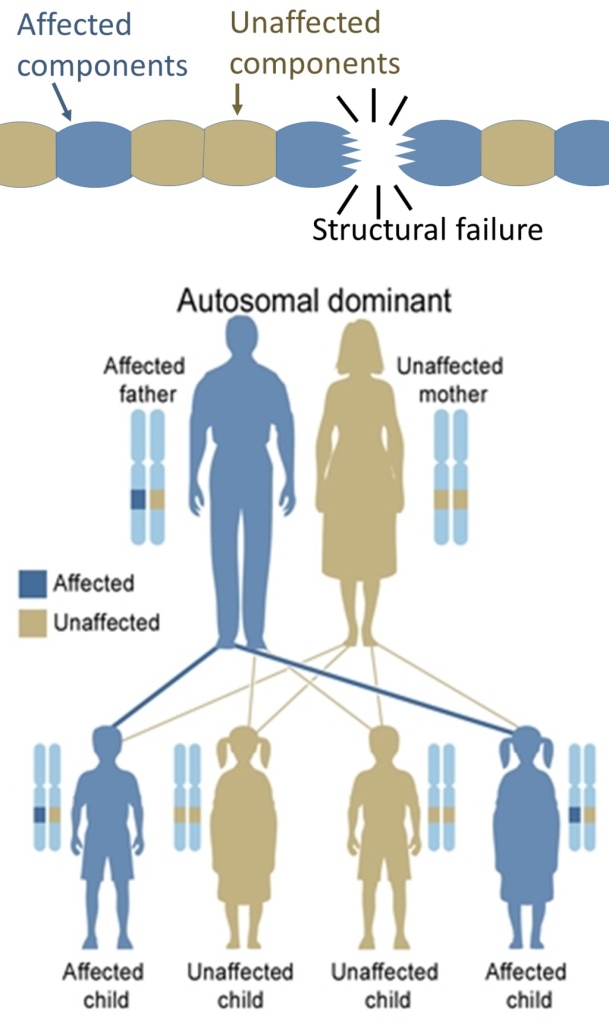

Main article: X-linked dominant
X-linked dominant disorders are caused by mutations in genes on the X chromosome. Only a few disorders have this inheritance pattern, with a prime example being X-linked hypophosphatemic rickets. Males and females are both affected in these disorders, with males typically being more severely affected than females. Some X-linked dominant conditions, such as Rett syndrome, incontinentia pigmenti type 2, and Aicardi syndrome, are usually fatal in males either in uteroor shortly after birth, and are therefore predominantly seen in females. Exceptions to this finding are extremely rare cases in which boys with Klinefelter syndrome (44+xxy) also inherit an X-linked dominant condition and exhibit symptoms more similar to those of a female in terms of disease severity. The chance of passing on an X-linked dominant disorder differs between men and women. The sons of a man with an X-linked dominant disorder will all be unaffected (since they receive their father’s Y chromosome), but his daughters will all inherit the condition. A woman with an X-linked dominant disorder has a 50% chance of having an affected fetus with each pregnancy, although in cases such as incontinentia pigmenti, only female offspring are generally viable.
X-linked recessive
Main article: X-linked recessive inheritance
X-linked recessive conditions are also caused by mutations in genes on the X chromosome. Males are much more frequently affected than females, because they only have the one X chromosome necessary for the condition to present. The chance of passing on the disorder differs between men and women. The sons of a man with an X-linked recessive disorder will not be affected (since they receive their father’s Y chromosome), but his daughters will be carriers of one copy of the mutated gene. A woman who is a carrier of an X-linked recessive disorder (XRXr) has a 50% chance of having sons who are affected and a 50% chance of having daughters who are carriers of one copy of the mutated gene. X-linked recessive conditions include the serious diseases hemophilia A, Duchenne muscular dystrophy, and Lesch–Nyhan syndrome, as well as common and less serious conditions such as male pattern baldness and red–green color blindness. X-linked recessive conditions can sometimes manifest in females due to skewed X-inactivation or monosomy X (Turner syndrome).[citation needed]
Y-linked
Main article: Y linkage
Y-linked disorders are caused by mutations on the Y chromosome. These conditions may only be transmitted from the heterogametic sex (e.g. male humans) to offspring of the same sex. More simply, this means that Y-linked disorders in humans can only be passed from men to their sons; females can never be affected because they do not possess Y-allosomes.[citation needed]
Y-linked disorders are exceedingly rare but the most well-known examples typically cause infertility. Reproduction in such conditions is only possible through the circumvention of infertility by medical intervention.
Mitochondrial
Main articles: Mitochondrial disease and Mitochondrial DNA
This type of inheritance, also known as maternal inheritance, is the rarest and applies to the 13 genes encoded by mitochondrial DNA. Because only egg cells contribute mitochondria to the developing embryo, only mothers (who are affected) can pass on mitochondrial DNA conditions to their children. An example of this type of disorder is Leber’s hereditary optic neuropathy.[31]
It is important to stress that the vast majority of mitochondrial diseases (particularly when symptoms develop in early life) are actually caused by a nuclear gene defect, as the mitochondria are mostly developed by non-mitochondrial DNA. These diseases most often follow autosomal recessive inheritance.[32]
See also: Gene therapy
The treatment of genetic disorders is an ongoing battle, with over 1,800 gene therapy clinical trials having been completed, are ongoing, or have been approved worldwide.[38] Despite this, most treatment options revolve around treating the symptoms of the disorders in an attempt to improve patient quality of life.
Gene therapy refers to a form of treatment where a healthy gene is introduced to a patient. This should alleviate the defect caused by a faulty gene or slow the progression of the disease. A major obstacle has been the delivery of genes to the appropriate cell, tissue, and organ affected by the disorder. Researchers have investigated how they can introduce a gene into the potentially trillions of cells that carry the defective copy. Finding an answer to this has been a roadblock between understanding the genetic disorder and correcting the genetic disorder.[39]
History
The earliest known genetic condition in a hominidwas in the fossil species Paranthropus robustus,with over a third of individuals displaying amelogenesis imperfecta.[40]
Towle I, Irish JD (April 2019). „A probable genetic origin for pitting enamel hypoplasia on the molars of Paranthropus robustus“ (PDF). Journal of Human Evolution. 129: 54–61. doi:10.1016/j.jhevol.2019.01.002. PMID 30904040. S2CID 85502058. Archived (PDF) from the original on 2023-06-04. Retrieved 2023-02-20.
Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J (February 2013). „Gene therapy clinical trials worldwide to 2012 – an update“. The Journal of Gene Medicine. 15 (2): 65–77. doi:10.1002/jgm.2698. PMID 23355455. S2CID 37123019.
Expanding the Boundaries of RNA Sequencing as a Diagnostic Tool for Rare Mendelian Disease
https://doi.org/10.1016/j.ajhg.2019.04.004
ORIGINAL ARTICLE| VOLUME 76, ISSUE 8, P777-783, AUGUST 2001
Prevalence and Patterns of Presentation of Genetic Disorders in a Pediatric Emergency Department
DOI:https://doi.org/10.4065/76.8.777
https://www.mayoclinicproceedings.org/article/S0025-6196(11)63220-5/abstract
Objective
To determine the prevalence and patterns of presentation of previously diagnosed and of suspected genetic disorders among pediatric emergency department (ED) visits to a hospital that serves an inner-city population.
Patients and Methods
A retrospective review of 15,258 pediatric (<18 years old) ED visits at Lincoln Medical and Mental Health Center was undertaken for visits that occurred between October 1998 and February 1999. Suspected genetic disorders, classified into chromosomal, single gene, multifactorial, and other syndromic categories, were recorded.
Results
Of 15,258 visits reviewed, 2839 visits (18.6%) were by patients who had known or suspected genetic disorders. Previously diagnosed genetic disorders were documented in 80 visits (2.8%). Of these, 69 visits (86.2%) were related to single gene disorders, 3 (3.8%) to chromosomal disorders, 6 (7.5%) to multifactorial disorders, and 2 (2.5%) to disorders in the “other” category. Of these 80 visits, 59 (74%) were associated with sickle cell disease. The remaining 2759 visits (97.2%) were associated with complaints or diagnoses that suggested the possibility of an underlying genetic disorder requiring further evaluation and diagnostic work-up.
Conclusions
Pediatric patients with known or suspected genetic disorders are frequently treated in EDs. Awareness of underlying genetic disorders facilitates diagnostic evaluation, treatment planning, and referral to a genetics clinic for counseling.ED (emergency department), LMMHC (Lincoln Medical and Mental Health Center)
Linked Article
- The Growing Impact of Genetics on Health Care: Do We Have Appropriate Educational Resources?Mayo Clinic ProceedingsVol. 76Issue 8
- Gene mutations as a cause of human disease.in: Sutton HE Harris MI Mutagenic Effects of Environmental Contaminants. Academic Press,New York, NY1972: 3-14View in Article
- The incidence of genetic disease in a university hospital population.Am J Hum Genet. 1973; 25: 237-246View in Article
- The frequency of genetic disease and congenital malformation among patients in a pediatric hospital.Can Med Assoc J. 1973; 108: 1111-1115View in Article
The frequency and financial burden of genetic disease in a pediatric hospital.Am J Med Genet. 1978; 1: 417-436 View in Article
Genetic aspects of admissions to a paediatric intensive care unit.Arch Dis Child. 1991; 66: 639-641 View in Article
Contribution of birth defects and genetic diseases to pediatric hospitalizations: a population–based study.Arch Pediatr Adolesc Med. 1997; 151: 1096-1103 View in Article
The impact of birth defects and genetic diseases [editorial].Arch Pediatr Adolesc Med. 1997; 151: 1082-1083 View in Article
- Nature and frequency of genetic disease.in: Rimoin DL Connor JM Pyeritz RE 3rd ed. Emery and Rimoin’s Principles and Practice of Medical Genetics. Vol 1. Churchill Livingstone,New York, NY1997: 31-34View in Article
Smith’s Recognizable Patterns of Human Malformation. 5th ed. WB Saunders Co,Philadelphia, Pa1997 View in Article
- Scriver CR Beaudet AL Sly WS Valle D The Metabolic and Molecular Bases of Inherited Disease. 7th ed. McGraw–Hill, New York, NY1995View in Article
- Stevenson RE Hall JG Goodman RM Human Malformations and Related Anomalies. Oxford University Press, New York, NY1993View in Article
Care–seeking patterns of inner–city families using an emergency room: a three–decade comparison.Med Care. 1996; 34: 1171-1179View in Article
- Family–genetic and psychosocial risk factors in DSM–III attention deficit disorder.J Am Acad Child Adolesc Psychiatry. 1990; 29: 526-533View in Article
- Neuropsy–chiatric disorders in the 22q11 deletion syndrome.Genet Med. 2001; 3: 79-84View in Article
Contribution of heritable disorders to mortality in the pediatric intensive care unit.Pediatrics. 1995; 95: 678-681View in Article
- OMIM: Online Mendelian Inheritance in Man. National Center for Biotechnology, Bethesda, Md2000Available at: www3.ncbi.nim.nih.gov/Omim/(Accessibility verified May 21, 2001.)View in Article
Anti-Kickback Statute, 42 U.S.C. § 1320a-7b (“AKS”), and the False Claims Act.
D. On April 6, 2022, HHS-OIG issued OIG Advisory Opinion No. 22-06, which addressed a request by another entity to Impfkristall free genetic testing and counseling services under certain circumstances (the “OIG Advisory Opinion)
Hinterlasse einen Kommentar