SHEDDING
Clinical protocol (Pages 67-69)
8.3.5. Exposure During Pregnancy or Breastfeeding, and Occupational Exposure
Exposure to the study intervention under study during pregnancy or breastfeeding and occupational exposure are reportable to Pfizer Safety within 24 hours of investigator awareness.
8.3.5.1. Exposure During Pregnancy
An EDP occurs if:
• A female participant is found to be pregnant while receiving or after discontinuing study intervention.
• A male participant who is receiving or has discontinued study intervention exposes a female partner prior to or around the time of conception.
• A female is found to be pregnant while being exposed or having been exposed to study intervention due to environmental exposure. Below are examples of environmental exposure during pregnancy:
• A female family member or healthcare provider reports that she is pregnant after having been exposed to the study intervention by inhalation or skin contact.
by inhalation or skin contact.
• A male family member or healthcare provider who has been exposed to the study intervention by inhalation or skin contact then exposes his female partner prior to or around the time of conception.
A male family member or healthcare provider who has been exposed to the study intervention by inhalation or skin contact then exposes his female partner prior to or around the time of conception.
HE IS EXPOSED BY INHALATION OR SKIN CONTACT AND THEN HE EXPOSES HIS PARTNER BY INHALATION OR SKIN CONTACT!
SO, WHAT’S IN THESE VIALS ?

https://karger.com/cop/article/12/3/944/822388/Ocular-Surface-Erosion-after-Suspected-Exposure-to
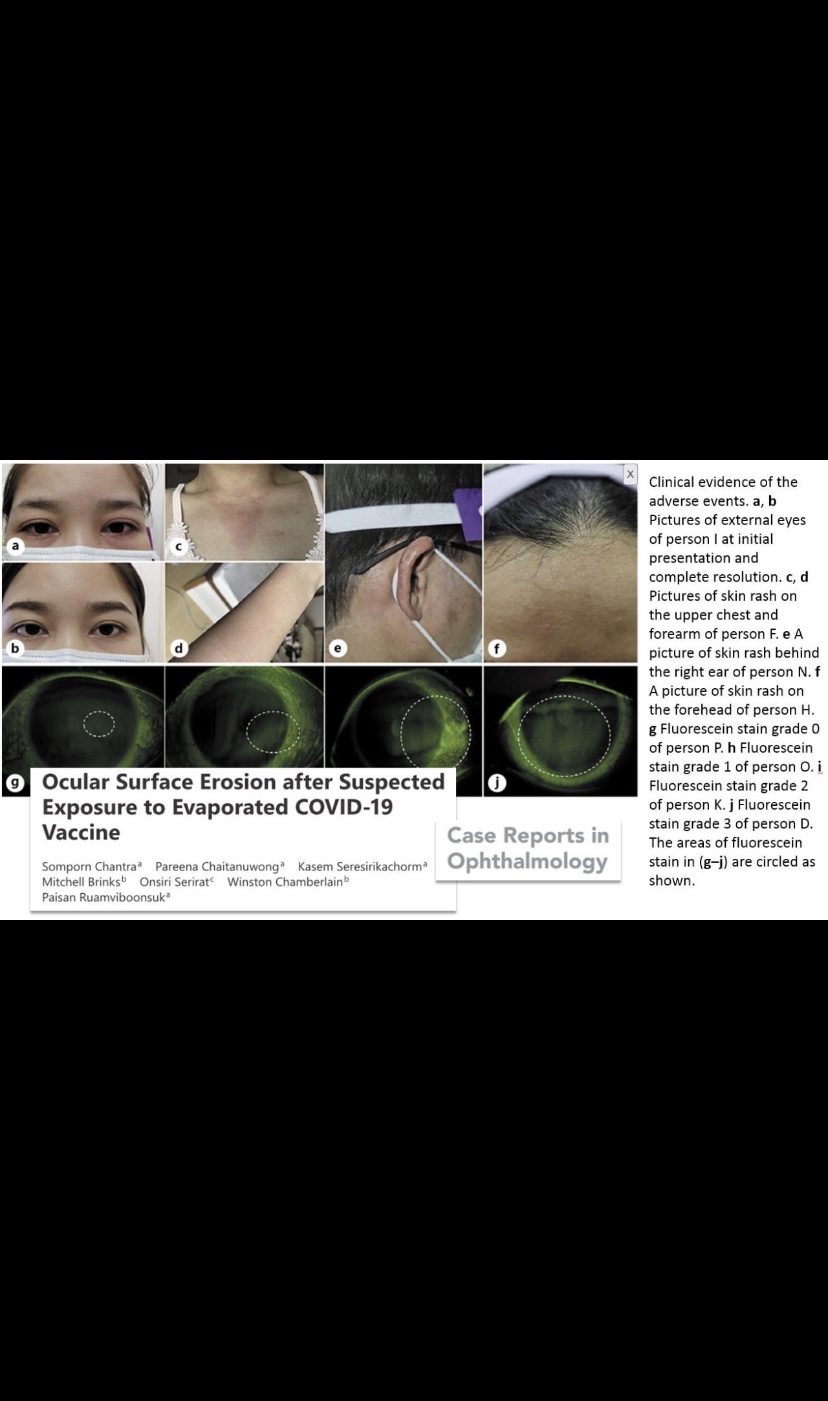
A total of 15 personnel had symptoms and signs of ocular surface erosion at the average time from the accident to the onset of 10.2 ± 7.1 h; 4 personnel also had skin rash. These personnel included all 13 persons who already worked in the rooms when the accident occurred and continued for additional 4–6 h and 2 personnel who presented in the rooms 1–2 h after the accident and stayed for 2–3 h. Proximity and timing suggest CoronaVac correlation with the ocular and skin reactions. Cautions should be taken to avoid broken vials, spills, and aerosolization of CoronaVac during the vaccination.
If EDP occurs in the setting of environmental exposure, the investigator must report information to Pfizer Safety using the Vaccine SAE Report Form and EDP Supplemental Form. Since the exposure information does not pertain to the participant enrolled in the study, the information is not recorded on a CRF; however, a copy of the completed Vaccine SAE Report Form is maintained in the investigator site file.
Follow-up is conducted to obtain general information on the pregnancy and its outcome for all EDP reports with an unknown outcome. The investigator will follow the pregnancy until completion (or until pregnancy termination) and notify Pfizer Safety of the outcome as a follow-up to the initial EDP Supplemental Form.
In the case of a live birth, the structural integrity of the neonate can be assessed at the time of birth. In the event of a termination, the reason(s) for termination should be specified and, if clinically possible, the structural integrity of the terminated fetus should be assessed by gross visual inspection (unless preprocedure test findings are conclusive for a congenital anomaly and the findings are reported).
Abnormal pregnancy outcomes are considered SAEs. If the outcome of the pregnancy meets the criteria for an SAE (ie, ectopic pregnancy, spontaneous abortion, intrauterine fetal demise, neonatal death, or congenital anomaly), the investigator should follow the procedures for reporting SAEs.
Additional information about pregnancy outcomes that are reported to Pfizer Safety as SAEs follows:
Spontaneous abortion including miscarriage and missed abortion•
Neonatal deaths that occur within 1 month of birth should be reported, without regard to causality, as SAEs. In addition, infant deaths after 1 month should be reported as SAEs when the investigator assesses the infant death as related or possibly related to exposure to the study intervention.
Neonatal deaths that occur within 1 month of birth should be reported, without regard to causality, as SAEs. In addition, infant deaths after 1 month should be reported as SAEs when the investigator assesses the infant death as related or possibly related to exposure to the study intervention.
Additional information regarding the EDP may be requested by the sponsor. Further follow-up of birth outcomes will be handled on a case-by-case basis (eg, follow-up on preterm infants to identify developmental delays). In the case of paternal exposure, the investigator will provide the participant with the Pregnant Partner Release of Information Form to deliver to his partner. The investigator must document in the source documents that the participant was given the Pregnant Partner Release of Information Form to provide to his partner.
8.3.5.2. Exposure During Breastfeeding
An exposure during breastfeeding occurs if:
• A female participant is found to be breastfeeding while receiving or after discontinuing study intervention.
• A female is found to be breastfeeding while being exposed or having been exposed to study intervention (ie, environmental exposure). An example of environmental exposure during breastfeeding is a female family member or healthcare provider who reports that she is breastfeeding after having been exposed to the study intervention by inhalation or skin contact. The investigator must report exposure during breastfeeding to Pfizer Safety within 24 hours of the investigator’s awareness, irrespective of whether an SAE has occurred. The information must be reported using the Vaccine SAE Report Form. When exposure during breastfeeding occurs in the setting of environmental exposure, the exposure information does not pertain to the participant enrolled in the study, so the information is not recorded on a CRF. However, a copy of the completed Vaccine SAE Report Form is maintained in the investigator site file. An exposure during breastfeeding report is not created when a Pfizer drug specifically approved for use in breastfeeding women (eg, vitamins) is administered in accord with authorized use. However, if the infant experiences an SAE associated with such a drug, the SAE is reported together with the exposure during breastfeeding.
8.3.5.3. Occupational Exposure
An occupational exposure occurs when a person receives unplanned direct contact with the study intervention, which may or may not lead to the occurrence of an AE. Such persons may include healthcare providers, family members, and other roles that are involved in the trial participant’s care.
The investigator must report occupational exposure to Pfizer Safety within 24 hours of the investigator’s awareness, regardless of whether there is an associated SAE. The information must be reported using the Vaccine SAE Report Form. Since the information does not pertain to a participant enrolled in the study, the information is not recorded on a CRF; however, a copy of the completed Vaccine SAE Report Form is maintained in the investigator site file.
IF ONLY INHALATION OR SKIN CONTACT WITH “STUDY INTERVENTION” CAN LEAD TO ADVERSE EFFECTS, HOW CAN THESE INJECTIONS BE CONSIDERED SAFE FOR PREGNANT OR BREASTFEEDING WOMEN, OR FOR BABIES
https://www.sciencedirect.com/science/article/pii/S2451929416300602


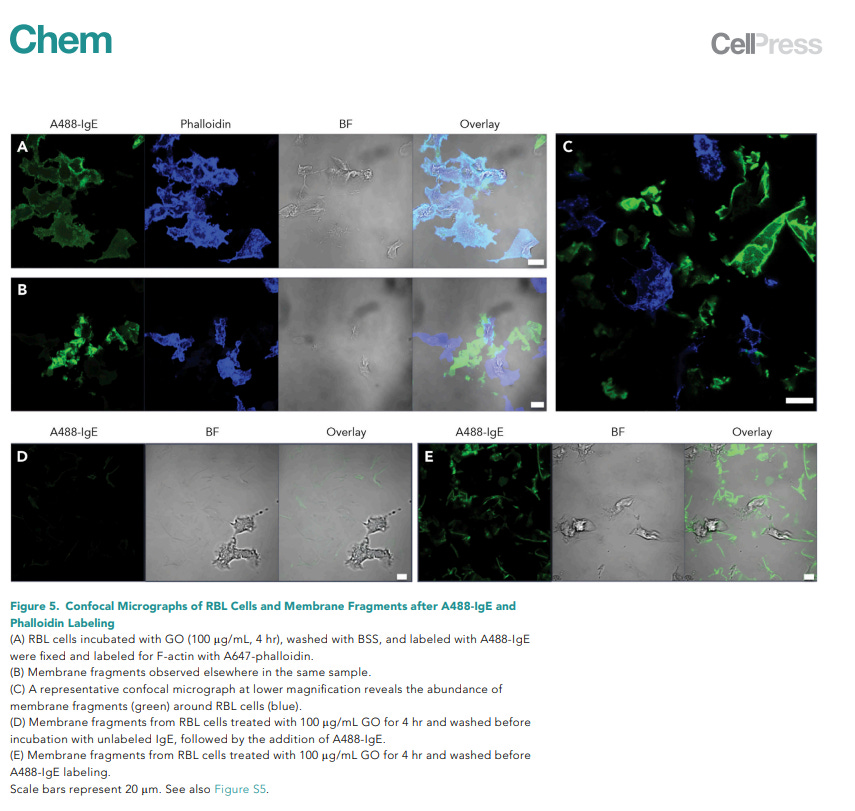
We and others have observed multiple GO-stimulated cellular responses occurring on the temporal scale of a few hours, such as vacuolization,23 PM permeabilization,23 PM ruffling and fragmentation, and PM receptor endocytosis. GO-induced Toll-like-receptor-based inflammatory responses and necrosis occur at a much longer incubation time and are likely linked to aforementioned short-term responses of PM disruption.21,22 The temporal progression of GO-stimulated cellular responses provides a clue to uncovering the molecular basis of these phenomena. Future studies will focus on understanding the cellular mechanisms underlying this membrane fragmentation and how the anomalous membrane behaviors relate to altered membrane signaling, cell motility, adhesion, polarity, and other cellular processes.7,25,26,45 Our findings reveal previously undescribed mammalian cellular responses to this foreign nanomaterial; although not cytotoxic, they likely reflect environmental stress.
Hinterlasse einen Kommentar